Aby zapoznać się z nagraniem referatu, należy skontaktować się z prof. Andrzejem Szewczykiem:
Water splitting for large-scale hydrogen production is a method for storing sustainable but intermittent energy sources. Oxygen evolution reaction (OER) through the water oxidation reaction provides low-cost electrons for the formation of hydrogen. OER is a complicated, sluggish reaction and a bottleneck for water splitting. Herein, first, a tetranuclear Ni complex with di(2-pyridyl) ketone (compound 1) has been synthesized. In the next step, OERs in the presence of compound 1 at pHs 3.0 and 7.0 have been investigated. The study attempts to answer the following questions for the metal complex during OER: (i) what is the true catalyst for OER in the presence of a Ni complex under neutral or acidic conditions? (ii) Why is low OER observed in the presence of a Ni complex under neutral or acidic conditions? The experiments show that the Ni-oxo cluster of γ-NiO(OH) is formed during OER in the presence of compound 1 at pHs 3.0 and 7.0. In addition, compound 1 is reduced on the counter electrode surface at pH 3.0 during OER. The reduced complex is characterized by Raman spectroscopy and electron paramagnetic resonance as a Ni(I) complex, which is unstable and decomposed after a few hours. Thus, a metal complex must be stable on the working electrode surface and the counter electrode surface for OER in a single-cell setup [1].
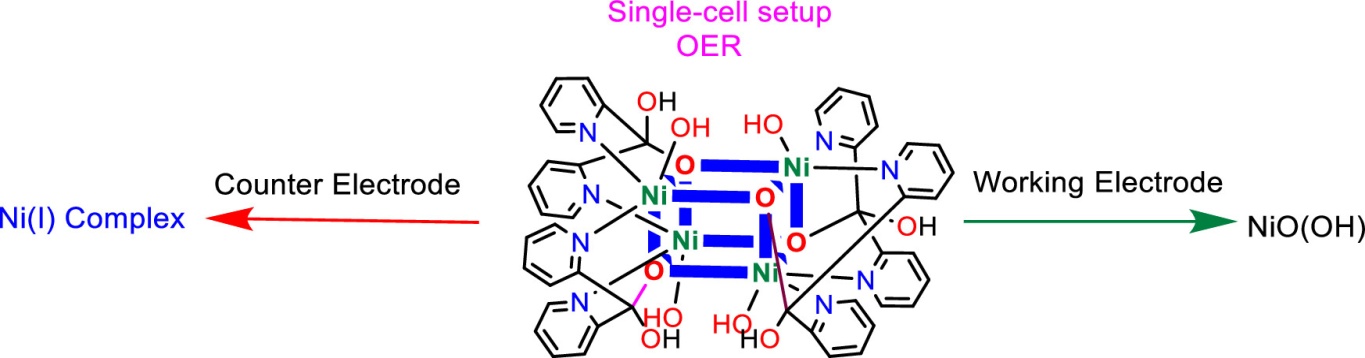
[1] Kalantarifard Shima, Akbari Nader, Aleshkevych Pavlo, Nandy Subhajit, Chae Keun Hwa, Najafpour Mohammad, ACS Applied Energy Materials 6 (2023) 3881
Wykład będzie prowadzony w języku polskim w sali 203, dostępna będzie również transmisja ZOOM - link podany jest na stronie IF PAN.
Informacje dotyczące sesji Zoom.
Lista terminów (Strona szczegółów wydarzenia)
- 06-12-2023 10:00 - 11:00